Like many global biopharmaceutical companies, the Sponsor was pouring millions per year into various patient recruitment tactics across their clinical trial portfolio. However, they struggled to understand which of these tactics were yielding positive results. This limited their ability to effectively allocate their recruitment budget and maximize return.
These blind spots were a result of known issues in the recruitment and
enrollment funnel. Specifically, research sites were burdened with multiple streams of inbound referrals and the need to aggregate and report recruitment and pre-screening information back to the Sponsor. This labor-intensive process multiplied the sites’ workload and detracted from their ability to pre-screen, screen, and enroll patients.
The inability to tie specific recruitment strategies to enrolled patients led to higher costs, slower enrollment, and the Sponsor’s uncertainty around which actions to take.
After running a systematic search for solutions, the Sponsor chose StudyTeam. StudyTeam is a recruitment and enrollment management solution that enables their trial teams to see which recruitment tactics are working months earlier than before.
Additionally, after activating StudyTeam’s Referral Partner Interface (RPI), referrals from the sponsor’s central recruitment campaigns were delivered directly into their sites’ workflow via StudyTeam for Sites, a system that sites use to manage patients from all recruitment sources.
The sponsor could view recruitment data across all sources and easily
tie enrolled patients back to their original source. This empowered trial
teams to make quick and actionable decisions that drove measurable and trackable recruitment ROI.
Centralized information
Sponsor was able to view enrollment progress from all sources in one place, including central recruiting campaigns, chart review activity at sites, and site-driven marketing and referral activities. This allowed the sponsor to divert spending toward reimbursing sites for additional hours of chart review, which was a more effective tactic for the
trial’s patient population. This brought the cost of recruitment from ~$100K per patient down to $11,109 per patient randomized.
End-to-End Data
StudyTeam’s ability to digitally connect the Sponsor with its sites enables sponsors to see the full picture of recruitment data in real-time vs. a small slice several weeks delayed.
Engaged Sites
Broad adoption by sites provided the Sponsor with reliable insights and the ability to take action with confidence. Visibility into global country-by-country pre-screening trends allowed the Sponsor to reallocate patients to sites in a faster-enrolling country after pre-
screening in a planned top-enrolling country plateaued. This saved the Sponsor 4 weeks of enrollment time with $0 additional spend.
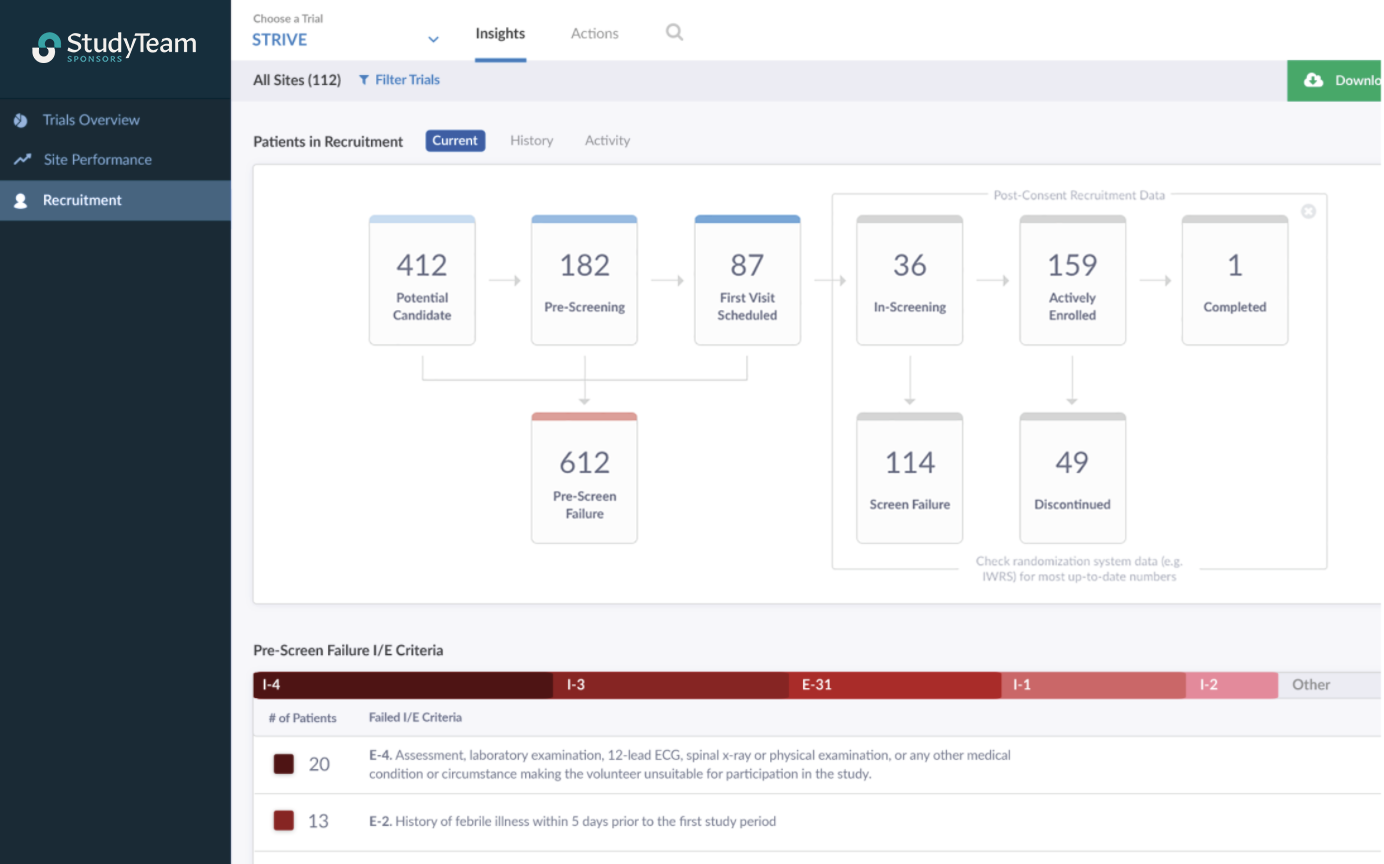
StudyTeam’s Sponsor Trial Dashboard and I/E criteria report
• StudyTeam’s Referral Partner Interface (RPI) integrates a sponsor’s central recruitment campaigns into the site’s workflow
• Pre-screening and screening trends in StudyTeam provide an early warning sign that enrollment might be going slower than planned
• StudyTeam Recruitment Source Analysis provides visibility into each recruitment source to track efficiency / ROI
Gain insights and control that you’ve never had before. A clinical trial enrollment software that both sponsors and sites will love.
.png?width=65&name=OST%20Transparent%20(1).png)
OneStudyTeam, a member of the Reify Health portfolio, provides the cloud-based platform StudyTeam to accelerate the development of new and life-saving therapies. StudyTeam brings research site workflows online and enables sites, sponsors, and other key stakeholders to work together more effectively using common technology. The suite of StudyTeam solutions reduces site burden and helps sites pre-screen and enroll more patients, provides sponsors with end-to-end visibility into recruitment activity across all channels, and guides sites in conducting the trial for patients who have been enrolled. StudyTeam is trusted by the largest global biopharmaceutical companies, used in more than 6,000 research sites, and is available in over 100 countries.
One mission. One team. That’s OneStudyTeam.
33 Arch Street 17th Floor Boston, MA 02110
Join Our Mailing List
Get OneStudyTeam news and clinical trial enrollment insights delivered directly to your inbox.
Copyright © 2024 OneStudyTeam, Inc. All Rights Reserved.