The sponsor was fairly confident that they knew the optimal sites and
key opinion leaders to enroll their rare disease patients. However, inaccurate feasibility estimates, difficulty obtaining consent, and
competition for limited numbers of patients threatened their ability to
enroll their trial on time.
Additionally, the sponsor knew that traditional central advertising
campaigns were not likely to accelerate enrollment timelines on
rare disease trials. Traditional tactics like activating more sites and
amending the protocol could possibly benefit enrollment, but cost and
uncertainty around ROI made those options less attractive.
The sponsor deployed StudyTeam's enrollment
performance management solution early in the site activation process.
Quick and widespread adoption by global sites gave the Sponsor
immediate insight into the numbers of candidate patients at each site,
pre-screening efficiency, I/E criteria analytics, and more.
With StudyTeam deployed across this critical rare disease trial, the Sponsor was able to focus its R&D capital, clinical operations team, and its sites on the work that would maximize efficiency and the probability of hitting their enrollment targets.
As a result, the sponsor intervened early by amending its protocol within ~2 months of first patient enrollment and completed enrollment 29 days ahead of schedule. Completing enrollment early saved it substantial amounts on unnecessary services spend and accelerated time to market, thereby improving revenue potential.
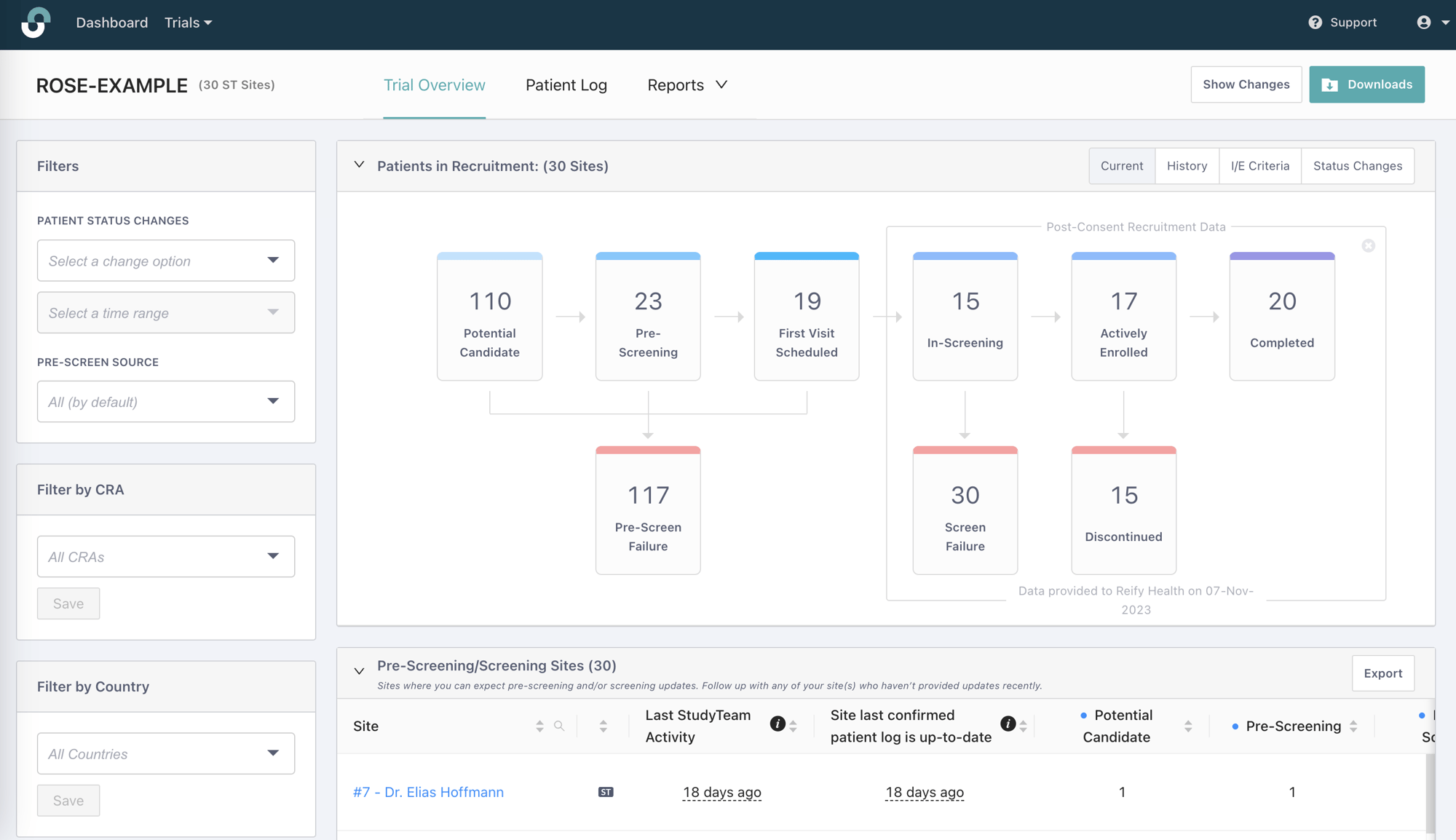
A sample Trial Dashboard illustrating the types of real-time data sponsors receive in StudyTeam.
• Visibility into the full recruitment and enrollment funnel beginning at the earliest stages of site activation
• Digital I/E Criteria tracking and analysis
• Digital patient source tracking and analysis
• User experience that motivates sites to do the work that moves patients through the recruitment and enrollment funnel
Gain insights and control that you’ve never had before. A clinical trial enrollment software that both sponsors and sites will love.
.png?width=65&name=OST%20Transparent%20(1).png)
OneStudyTeam, a member of the Reify Health portfolio, provides the cloud-based platform StudyTeam to accelerate the development of new and life-saving therapies. StudyTeam brings research site workflows online and enables sites, sponsors, and other key stakeholders to work together more effectively using common technology. The suite of StudyTeam solutions reduces site burden and helps sites pre-screen and enroll more patients, provides sponsors with end-to-end visibility into recruitment activity across all channels, and guides sites in conducting the trial for patients who have been enrolled. StudyTeam is trusted by the largest global biopharmaceutical companies, used in more than 6,000 research sites, and is available in over 100 countries.
One mission. One team. That’s OneStudyTeam.
33 Arch Street 17th Floor Boston, MA 02110
Join Our Mailing List
Get OneStudyTeam news and clinical trial enrollment insights delivered directly to your inbox.
Copyright © 2024 OneStudyTeam, Inc. All Rights Reserved.