See how StudyTeam works
Join a free, 30-minute walk-through of our patient enrollment management tool. You’ll see how to implement easy-to-use features and practices to enroll patients and share recruitment insights faster.
Learn how to increase enrollment efficiency with StudyTeam by:
- Streamlining administrative tasks
- Supporting patient follow-ups and updates
- Strengthening visibility of data and insights
- Simplifying communication between sites and sponsors
Accelerate your research studies
The faster you enroll patients, the sooner you develop the therapies they need. Check out the platform used by some of the largest biopharmaceutical companies to complete patient enrollment sooner while staying up-to-date with data coming from clinical trial sites.
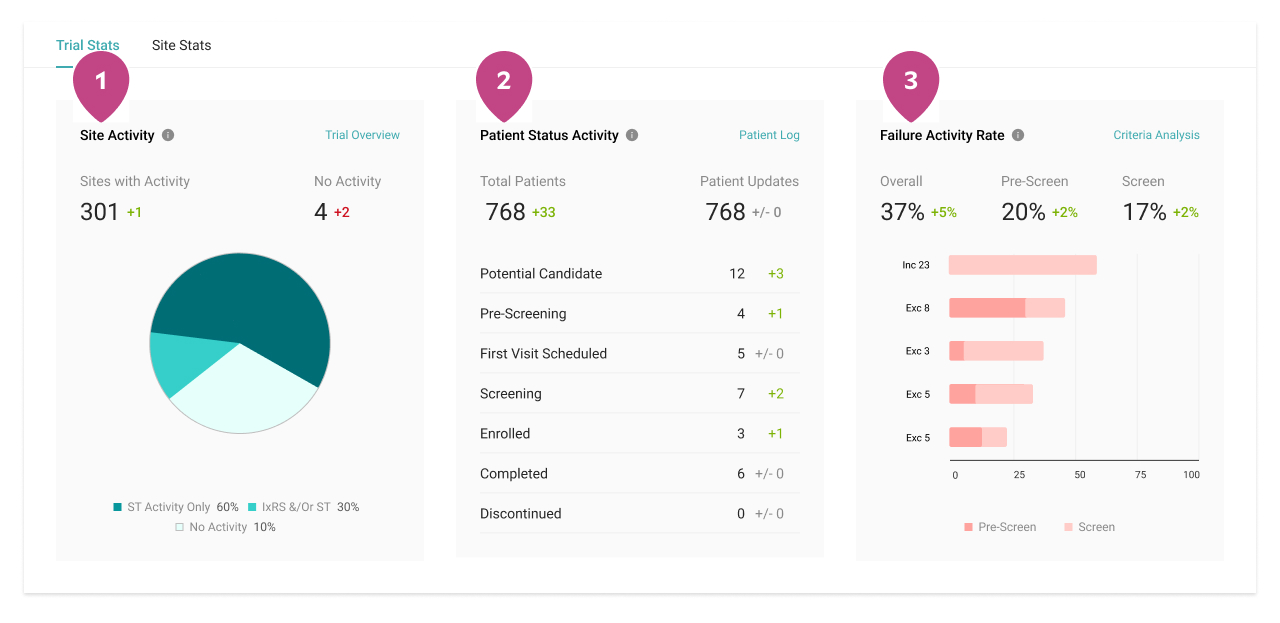
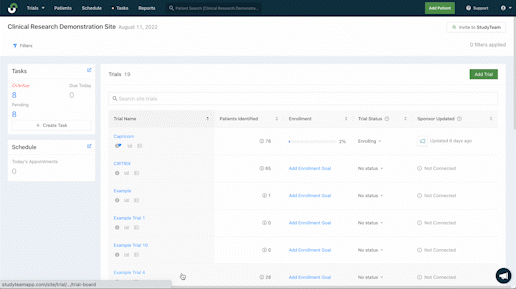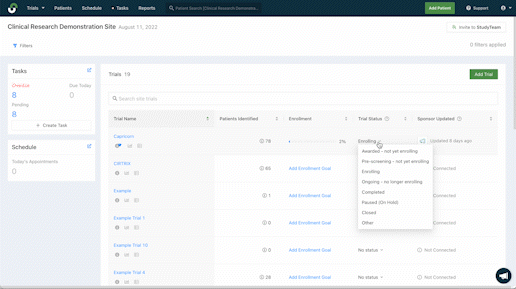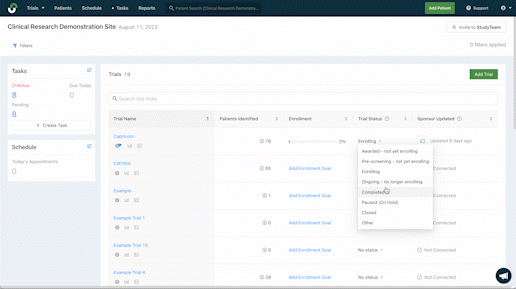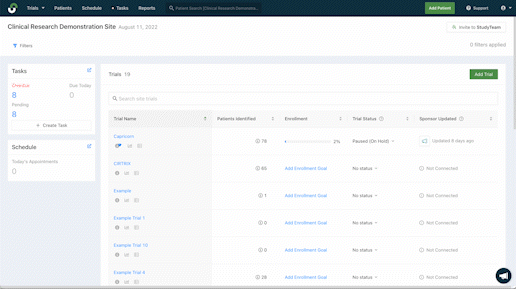
Improve management across studies
Quicker patient enrollment processes mean more time to provide critical care. Explore the features that more than 5,000 clinical trial sites rely on globally to eliminate inefficiencies throughout recruitment and enrollment.
Testimonial
“I value the ability to assess earlier in the funnel along the patient journey where the gaps are. Study managers can analyze aggregated data around specific entry criteria through StudyTeam to decide where to pivot.”
Neha Londoño - Director of Global Clinical Trial Diversity & Inclusion at Seagen
.png)

